Suplementação Oral de Silício e seu Impacto na Qualidade do Cabelo

Nonychosine V®: Complexo fortalecer de unhas
19 de janeiro de 2018
Suplementação dietética com peptídeos específicos de colágeno tem um efeito benéfico dependente do índice de massa corporal na morfologia da celulite
17 de maio de 2018Suplementação Oral de Silício e seu Impacto na Qualidade do Cabelo
Por Luciana Nakanishi, Bruna Bombonatti, Letícia Muller, Ricardo Villa, Maria Valéria, Valcinir Bedin e Ana Carolina Villa.
INTRODUCTION
Hair is unquestionably one of the most important personal attributes in all cultures, and, therefore, its characteristics are of great psychological importance, justifying all the studies focusing on how to conserve and promote hair quality. In this particular, is fundamental to consider that human hair has an outer protective layer, which surrounds the cells of the highly keratinized cortex and a structural hierarchy of disulfide bonds that has influence on the mechanical properties of hair.
Changes in growth and hair quality can be induced by protein malnutrition or low intake of oligoelements and vitamins and environmental aggression. These deleterious effects are manifested in the form of difficult
combing, brittleness and dryness.
In its turn, silicon (Si) is a well known and ubiquitous component present in several tissues of human body and is found at 1-10 ppm in hair and nails. Additionally, silicon accumulates in the stratum corneum of epidermis and in hair cuticle.9 Recently, it was confirmed that the phanars, hair and nails are more rich in silicon than the remaining parts of the skin and this element accumulates more intensely in the outer layer of the epidermis and in the cuticule of hair (Table 1), wich explains the usage of silicon in the treatment of alopecia since the middle of last century. Further, from his results, Fregert raised the question that silicon levels strongly contributes to the solidity and the high resistance of keratinous tissues and plays a role as a barrier. Indeed, it has been shown that silicon can improve the skin barrier of normal skin and prevents premature skin aging and psoriasis progression.
In animals, deficit of bioavailable silicon can be bound to severe diseases (arteriosclerosis, osteoarthritis, hypertension and aging processes). Beyond that, silicon deficiency in human body has been connected to many degenerative diseases (e.g., Alzheimer’s) and tissue aging processes, and that the normal silicon blood plasma
concentration varies from 5 to 20 micromoles, we propose that silicon supplementation could be considered.
The silicon absorption is considerably higher in the form of silanol as shown by a silicon study and osteogenesis. Furthermore, toxicological studies referred to the soluble silicon derivative as safe, with no genotoxic for use in living beings.
Objective of the study
Our primary objective of this study was to evaluate the correlation between metric parameters and laboratory assessments of patients undergoing oral supplementation of silicon.
Our secondary objective was to describe the subjective results of the hair quality after oral supplementation of silicon.
MATERIAL AND METHODS
We conducted an uncontrolled longitudinal study in wich our intervention was the supplementation of silicon for five months.
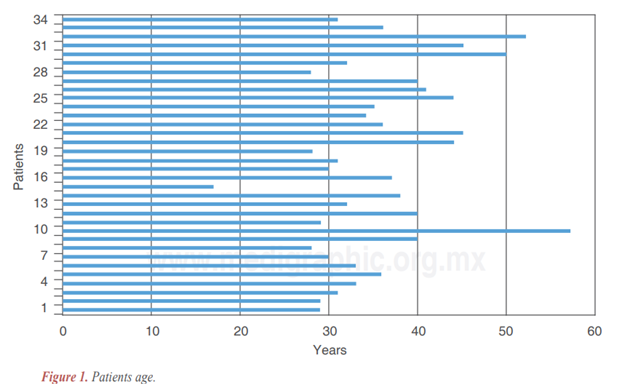
All of our 34 patients were women aged between 17 and 57 years, as displayed in Figure 1.
At day zero, we shaved the hair of an area equivalent to 1 cm² on the occipital protuberance of each patient. After this procedure, we measured three times at each moment (in order to validate the data) the hair length at intervals of 30 days for 3 months and obtained medium growth rate for each participant before the treatment was started. After these first three months, every patient was submitted to a new trichotomy and we re-started monthly measures, but, from this moment on, they were receiving the dose of 30 mg of orthosilicic acid in the form of OSA (orthosilicic acid) stabilized in marine collagen at a dose of 600 mg over five months. This dosage was divided in two capsules of 300 mg taken twice a day, at least two hours after meals for better absorption of orthosilicic acid.
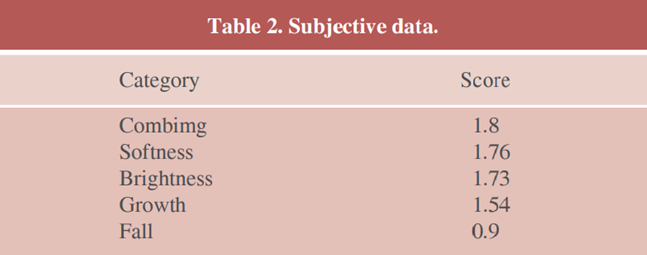
Patients participating in the study answered a subjective questionnaire about their hair focusing the following parameters: smoothness, shine, growth, and fall. They were instructed not to change the diet, daily activities and medications. Women who used any medication that could alter the variables addressed in the study, such as vitamins and other supplements were excluded. If they were using contraceptives, they had to continue and, if they didn’t, they couldn’t start until the end of the research. They attributed the following score, taking into account the grade of improvement of each parameter: significant improvement (2), moderate improvement (1) and no improvement (0). Medium scores were calculated and are displayed in the next section.
When exposition to physical or chemical treatments occurs, hair strand may exhibit damage in its structure, and therefore, a change occurs in the protein composition and resistance. In order to evaluate hair resistance, we used a validated assay that consists in submitted hair sample to a physical aggression (ultrasonic wave) in a tube with water and assess how much protein that hair sample looses to the water. The whole process is as follows: to accomplish the quantification of protein loss were diluted 100 mg of hair in 15 mL of distilled water in a test tube. This sample was subjected to ultrasonic bath for 40 minutes. After this protein extraction procedure, the suspension obtained was filtered and an aliquot of 2.0 mL of the filtrate of each sample was removed and subjected to protein quantification assay.
Spectrophotometry was the technique we have chosen to evaluate protein concentration in each sample. Our results of absorbance readings were plotted in the calibration curve. This curve uses albumin as a standard.
Statistical analysis
Our data concerning to hair growth were submitted to t-test, since our study were an uncontrolled longitudinal one and, with p < 0.000208438, we assumed that hair growth after treatment has increased (we rejected H0 =
H1 and accepted H0 H1).
In the same way, our absorbance data of hair samples exhibited significative improvement in hair resistance after treatment, with a p < 0.00283598, we assumed that hair resistance after treatment has increased (we rejected H0 = H1 and accepted H0 H1).
RESULTS
Our subjective data are as follows (Table 2):
As we can see, features related to hair texture received the highest score, and, in total, 83% of the volunteers reported a general improvement in the hair quality during treatment. Although growth was not the highest score, it was a feature with a very good response to treatment, as we can see below in our objective data (Table 2).
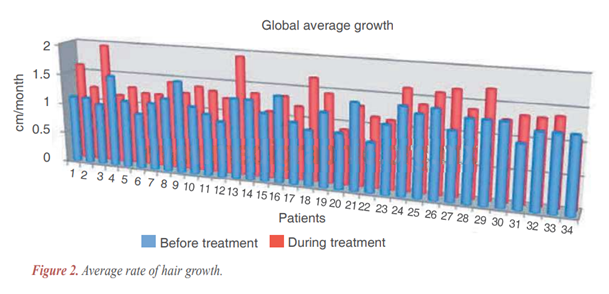
In (Figure 2), we display the average rate of hair growth (cm/month). In blue, before treatment and, in red, during treatment. This chart exposes precisely our results:
- In 79.4% of subjects (27/34), hair growth has increased (as mentioned above, with significance as described in statistical analysis).
- Comparing medium growth rate before and during treatment, there was an increase of 37.6%.
Protein loss
When we compared protein loss of samples obtained before and after treatment, we revealed that hair was more resistant after treatment and lost 84.2% less protein when submitted to ultrasound bath.
DISCUSSION
There is a correlation between the data found in animals regarding the diffusion of silicon to the target organs. In the blood and extra-vascular liquid, silicon is present physiologically in the hydrated orthosilicic acid form and non disassociated by the pH of the organism. In a mostly free form (> 98%), silicon of biological liquids, unbound to protein, is easily diffusible which allows for an even distribution in the blood between the plasma and the erythrocytes in the range of 265 g/L. Literature indicates that, following administration of silicon derivatives, silicon blood level rises, which bears witness to the possibility of absorption. In man and superior animals, the major path of elimination of silicon is the renal path with a clearance in man of 90 mL/min and a fractional excretion of the order of 90%. The resorption of filtered silicon is minimum.
The skin, mucous membranes and the connective tissue are the most rich in silicon. High concentrations (12-16 mg/kg) are also recovered in the aorta, trachea and the tendons. However, the parenchymal tissue shows weaker levels (2-10 mg/kg) with an exception for the lungs, which as a result of the constant exposure by inhalation of dusty minerals, contains quantities of silicon often very important. As the major excretion organ, the kidney is also very rich in silicon.
In the last 30 years, one of the most remarkable cont ributions to silicon knowlodge was the demonstration of the implication of silicon in bone growth and in the development of cartilage, articulation and other connective tissue, skin included. Specifically, it was shown that silicon intervenes in the synthesis of collagen and proteoglycans, as well as, in the early stage of bone mineralisation. The important silicon content of these tissues is linked to the silicon-polysaccharide interaction which makes silicon a compound of glycosaminoglycans and polyuronides. For example, in the umbilical cord the presence of 330 to 554 g of silicon per gram of hyaluronic acid, chondroitines sulfates, dextranes-sulfates and purified heparin is reported.
With embryonic bone or cartilage in culture, one observes growth which is a lot more rapid in a silicon enriched medium in comparison to that of a medium poor in silicon. The difference growth rate is correlated to the strong increase in the collagenic content and even more to those contents of polysaccharides of proteoglycans of the fundamental substance.
In this way, isolated chondrocytes confirms the stimulating effect of silicon on collagen synthesis without associated cellular proliferation, indicating that the site of action of silicon is on the synthesis of collagen and on the prolylhydroxylase intracellular. So, it was demonstrated, both in vitro and in vivo that the prolylhydroxylase, associated to collagenic synthesis, only reaches its optimal activity in the presence of sufficient silicon concentration.
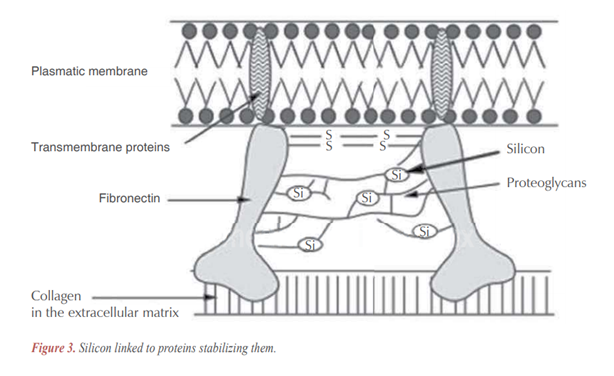
Silicon, besides its role in the organic matrix of connective tissue,9,23-25 seems to play an equally important role in their structure, providing connecting links of different polysaccharide and polyuronic hydrogen chains between them or with proteins (Figure 1).
Silicon is found in skin at a rate of 18 to 23 g per gram and, since collagen is the most abundant protein in the skin (making up 75 percent of its mass) silicon plays an important role stabilizing skin main components and prolylhydroxylase. Particularly, collagen type 1 contributes to skin integrity, including those sheets involving and protecting the follicle.
Hair, for its turn, contains 90 g of silicon in each gram (as mentioned in our introduction) and, probably, this element helps to keep its structure made of amino acids, named keratin, tight.
Silicon bioavailability in food is very low and that silicon decreases with aging and ongoing menopause process. In this particular, very few foods contains expressive amounts of silicon, such as grains, especially oats, barley, rice brain, wheat bran, cereal products, and plant-based foods, and it makes very difficult to obtain the minimum dosage of 2 to 5 mg/day. However, these grain products use the edible caryopsis of the cereal, whereas Si (phytolithic Si) is found almost solely in the husk. Hence, the Si content of cereal-based foods is mainly attributable to crosscontamination or to the purposeful addition of husk during processing. One cereal product, however, uses the macerated husk during its manufacture, namely beer. Not surprisingly, therefore, the Si content of beer appears to be high.
Silicon levels tend to be higher in foods derived from plants than foods from animal sources. Asians and Indians have much higher silicon intakes than do Western populations as a result of their higher intakes of plantbased foods.
Our study contributes to the established data mentioned above, focusing on effects of silicon in human body, particularly, in hair. First, we could appreciate an increase in hair growth rate with a high level of statistical confidence and, though our sample was small, our data, when submitted to statistical analysis, did prove
its beneficial role. Second, our laboratory assay was a validated one and unquestionably addressed the potential of silicon as a resource of strength to hair. Finally, none of our patients complained of side effects due to silicon administration, confirming available data on safeness and security of silanol.
These effects we observed could be linked to the strengthening of hair sheets and strand, since both of them have a high level of silicon and its role as factor contributing to skin resistance is well established.
This work focus was aimed at female patients, due to convenience for the metric monitoring during the eight months of study, insofar as hair has been shaved at baseline, at the end of third month, and at the end of the fifth month again. Therefore, we have chosen occipital protuberance area for tricotomy (a hidden one), and corresponding hair strand could not be cut during the study to not interfere with the analysis of capillary growth.
Ultimately, our suggestion to researchers concerned with hair quality and its relation to silicon would be to add new variables to the equation, such as silicon serum levels before and after treatment and, of course, enlarge the sample, giving even more reliability to future studies.
CONCLUSION
Our results allow to consider that silicon represents an important option concerning to hair treatment, leading both to increasing in hair growth and a more resistant strand, confirming silicon role as an effective therapy. Further studies must be conducted ir order to adress the exact relation between other relevant aspects, such as dose, therapeutic results, age of patients and serum levels of silicon.